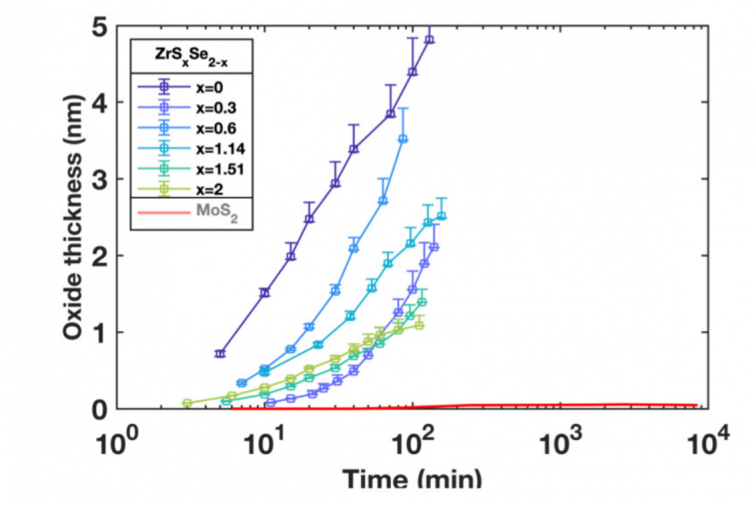
A thorough understanding of the processing and properties of native oxides is essential for designing semiconductor devices. This is no less true for nanomaterials than it is for legacy semiconductors such as silicon, for which control and understanding of the native oxide was a seminal achievement of 20th century materials science. Layered transition metal dichalcogenides nominally have inert, fully-passivated surfaces, but it is well-known that this is an oversimplification and that many TMDs oxidize readily. Here we report on experiments and simulations that reveal the rate and mechanism of oxidation of MoS2 and ZrSxSe2−x alloys in laboratory conditions. We find that MoS2 surfaces are indeed stable, with no oxidation for up to a year exposure. In contrast, ZrSxSe2−x alloys oxidize spontaneously and the oxide growth is not self-limited. Oxidation proceeds by Zr-O bond switching that collapses the van der Waals gaps, and is facilitated by progressive redox transitions of the chalcogen. In contrast, the MoS2 surface remains inert due to energetically-unfavorable oxygen adsorption. Our results provide quantitative and conceptual guidance for designing and processing semiconductor devices based on MoS2 and ZrSxSe2−x, and identify the atomistic-scale mechanisms of bonding and phase transformations in layered materials with competing anions.
Paper available at: https://arxiv.org/abs/2007.00117