Research Areas
2025
Selective Control of Exciton and Trion Emission Using Metasurfaces
What we did
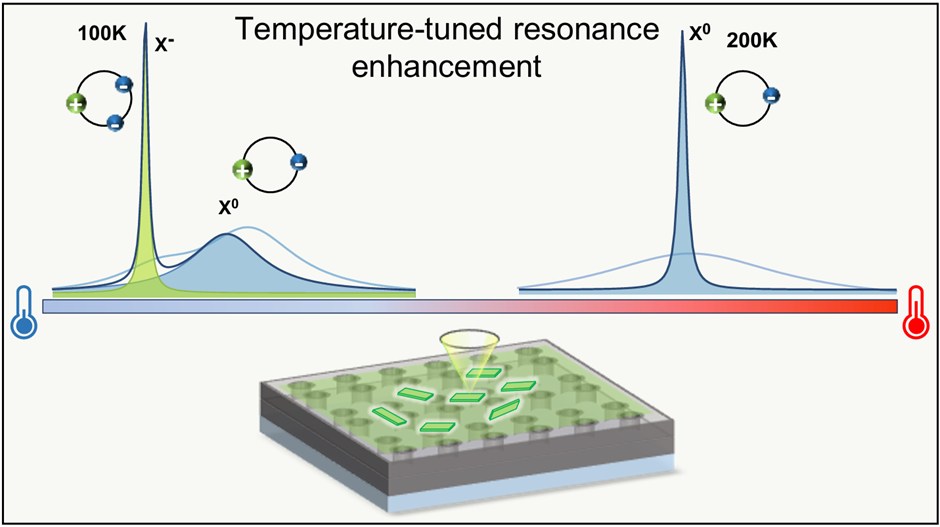
We developed a photonic strategy to selectively control neutral exciton (Xo) and trion (X⁻) emission in colloidal CdSe nanoplatelets (NPLs). By integrating these two-dimensional semiconductor emitters with a dielectric metasurface resonator (MSR) supporting high-Q guided-mode resonances, we engineered the light–matter interaction to deterministically enhance specific excitonic states. Instead of relying on irreversible photo-charging or chemical doping, we used temperature and polarization as reversible control knobs to tune and route excitonic emission.
Key findings
- Coupling NPLs to the MSR enabled selective Purcell enhancement of X⁻ and Xo emission at different temperatures through controlled spectral alignment.
- We observed up to 4.2× enhancement of X⁻ emission and 3× enhancement of Xo emission, accompanied by linewidth narrowing down to ~2.5 meV.
- The coupled emission exhibited Fano-like asymmetric line shapes, providing clear spectral signatures of coherent interaction between excitonic transitions and guided photonic modes.
- Time-resolved photoluminescence measurements revealed reduced radiative lifetimes, confirming Purcell enhancement with a factor of ~1.6 under resonant conditions.
- An anisotropic metasurface design enabled polarization-selective emission control, allowing Xo and X⁻ emission to be preferentially routed into orthogonal polarization channels.
Why it matters
Xo and X⁻ play distinct roles in quantum and optoelectronic devices, but controlling them independently in colloidal systems has remained challenging. Our work introduces a reversible, non-invasive, and scalable approach to excitonic state selection using photonic resonators. The ability to independently control excitonic state and polarization opens new opportunities for polarization-encoded quantum light sources, low-threshold excitonic lasers, and integrated quantum photonic platforms—without modifying the emitter chemistry.
For more information: Sharma, Komal, Nitish Kumar Gupta, Venkatachalam P, Shankar Kumar Selvaraja, and Jaydeep K. Basu. “Mode-and Polarization-Selective Control of Exciton and Trion Emission in Colloidal Nanoplatelets Coupled to Guided Metasurface Resonators.” ACS Photonics (2026). https://doi.org/10.1021/acsphotonics.5c01774
Emergent Jamming Dynamics of Interfacial Nanoparticles in Polymer Blends Undergoing Phase Separation
Understanding how nanoparticles organize and dynamically evolve at soft material interfaces is essential for designing advanced functional materials. In polymer blends undergoing phase separation, nanoparticles can migrate to the interface between immiscible polymer phases, where they assemble, and under certain conditions, become dynamically arrested or “jammed.” In our recent study (https://doi.org/10.1021/acs.macromol.5c02114), we directly probe the microscopic dynamics of such interfacial nanoparticles and reveal how their collective jamming controls the kinetics of phase separation in polymer blends.
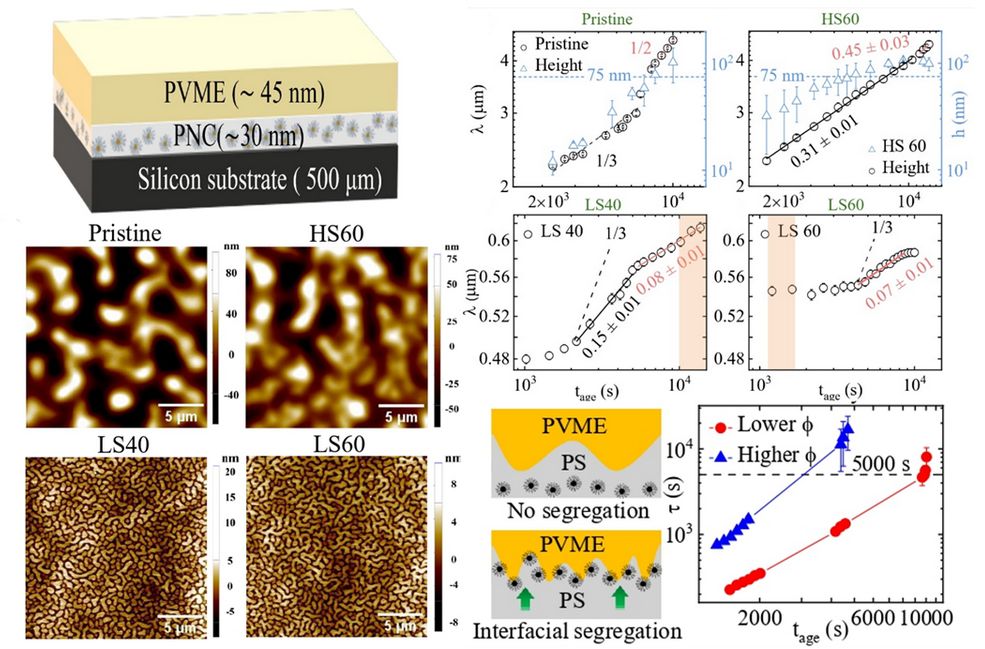
To achieve this, we developed a novel bilayer polymer geometry that enables selective probing of nanoparticles confined at buried interfaces. By combining Atomic Force Microscopy (AFM) with grazing-incidence X-ray photon correlation spectroscopy (XPCS) in standing-wave geometry, we were able to track, in situ and in real time, the nanoscale motion of polymer-grafted gold nanoparticles localized at the interface of phase-separating polymer films. This approach allowed us to directly visualize the temporal evolution of nanoparticle mobility and uncover the emergence of jamming behavior during phase separation—an experimental feat that has remained elusive until now.
Our measurements reveal a striking coupling between nanoparticle dynamics and macroscopic phase separation kinetics. At early times, interfacially active nanoparticles display enhanced mobility driven by the evolving polymer interface. As phase separation proceeds, increasing particle accumulation at the interface leads to a dramatic slowdown in dynamics, ultimately culminating in a jammed state. This dynamic arrest coincides with the onset of arrested phase separation in the polymer blend, establishing a direct microscopic link between nanoparticle jamming and macroscopic morphological control. By tuning particle grafting density and loading, we demonstrate systematic control over the timescale of jamming and, consequently, over the characteristic length scales of the resulting phase-separated structures.
Beyond advancing fundamental understanding, these findings offer practical pathways for materials design. Controlled interfacial jamming of nanoparticles enables precise tuning of domain sizes, opening opportunities for fabricating nanoporous materials, functional membranes, and structured polymer composites. More broadly, our work establishes a powerful experimental framework for probing nonequilibrium dynamics at buried soft interfaces, providing new insights into how nanoscale organization can dictate large-scale material properties.
For more information, see Biswas, A., Das, N., Swain, A., Anthuparambil, N. D., Mendez, N. F., Kulkarni, S., Keller, T. F., Sprung, M., Kumar, S. K., and Basu, J. K. (2026). Emergent Jamming Dynamics of Interfacial Nanoparticles in Polymer Blends Undergoing Phase Separation. Macromolecules. https://doi.org/10.1021/acs.macromol.5c02114
Microscopic insight into HIV fusion peptide-mediated dehydration and packing regulation in membranes

The human immunodeficiency virus (HIV) continues to pose a significant global health burden. A critical step in its life cycle is the fusion of the viral membrane with that of a host cell—a process mediated by the gp41 fusion peptide, a component of the viral envelope protein complex. This peptide acts as a molecular lever, disrupting the host membrane to initiate viral entry. In our recent study (https://doi.org/10.1016/j.bpj.2025.06.023), we present a detailed biophysical characterization of this fusion event, offering new insights into the molecular strategy employed by HIV.
The accompanying cover image (Cover image of August 5, 2025), rendered using the 3D modeling software Blender, visually represents the key findings of our study. It illustrates the gp41 fusion peptide (depicted as a helical structure) inserting into the lipid bilayer of the host cell. During this interaction, water molecules (shown as blue spheres) are expelled from the membrane surface, while the surrounding lipids are driven into a more tightly packed arrangement. This combination of membrane dehydration and increased lipid ordering forms the central mechanism by which gp41 promotes membrane fusion. Our conclusions are based on complementary biophysical techniques. Neutron reflectivity enabled precise quantification of hydration changes at the membrane interface, while fluorescence lifetime imaging microscopy (FLIM) provided a measure of lipid packing density. Together, these methods allowed us to reconstruct a detailed molecular view of the fusion process.
Importantly, we observed that gp41 exhibits enhanced activity in membranes characterized by lateral heterogeneity and disorder—conditions that mimic the nanodomains found in biological membranes. Interestingly, the introduction of negatively charged lipids attenuated the peptide’s ability to induce dehydration and packing, despite its continued penetration into the membrane. This suggests a nuanced dependence of fusion activity on membrane composition and charge properties. These findings have broader implications for antiviral strategies. While current HIV therapies primarily target post-entry stages of the viral lifecycle, our work points toward the possibility of interfering with the initial fusion event. Agents that stabilize membrane hydration or prevent excessive lipid packing may serve as viable candidates to inhibit viral entry.
Furthermore, the fusion mechanism uncovered here may not be unique to HIV. Several other enveloped viruses, including influenza and SARS-CoV-2, utilize similar fusion peptides for host entry. As such, the principles identified in this study could inform the development of broad-spectrum antiviral interventions with potential relevance beyond HIV.
Unraveling Direct Correlations between Membrane Nanodomain Reorganization and Antimicrobial Resistance Evolution in Bacterial Cells
Antibiotics are essential for treating bacterial infections; however, their excessive and improper use has led to the rise of antimicrobial resistance. Generally, a key driver of this resistance is the exposure of bacteria to sub-lethal concentrations of antibiotics, which often enables them to develop resistance against the very drugs meant to eliminate them. Given that many antibiotics exert their effects by targeting the bacterial membrane-either directly or indirectly-it becomes crucial to understand how membrane structure and dynamics respond during this evolutionary process.
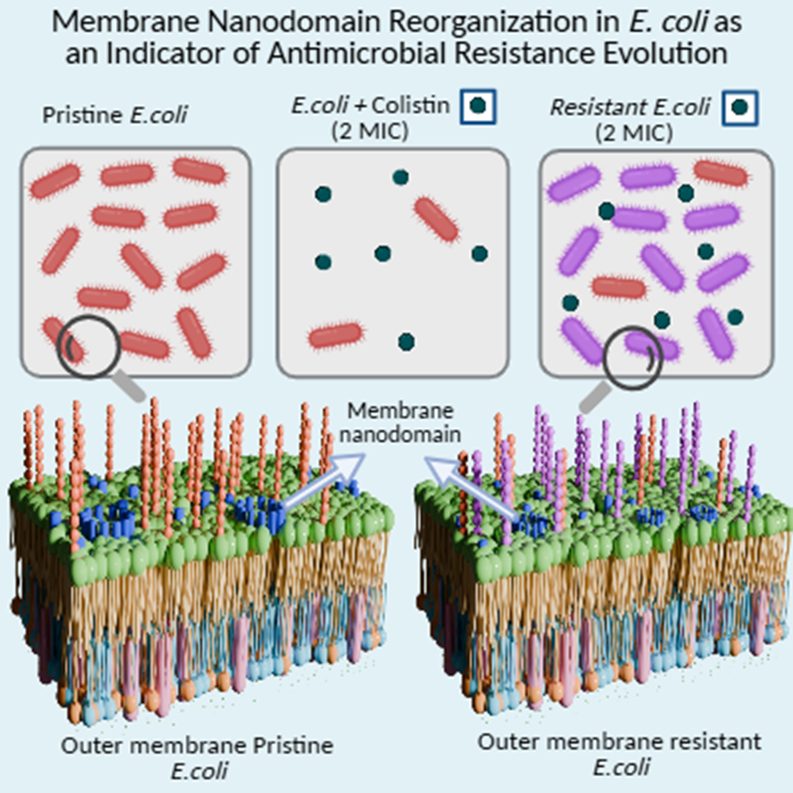
To explore this, we exposed live E. coli to progressively increasing concentrations of colistin, an antibiotic of last resort. Over a short period of time, the bacteria adapted to survive not only at the minimal inhibitory concentration but also beyond it. Gene expression analysis through RT-PCR revealed that resistance is accompanied by the upregulation of pmrAB and phoPQ, genes known to mediate modifications in lipopolysaccharides, thereby altering membrane characteristics.
To uncover the underlying membrane adaptations associated with this resistance, we employed super-resolution Stimulated Emission Depletion (STED) nanoscopy coupled with Fluorescence Correlation Spectroscopy (FCS). STED-FCS enabled us to probe the dynamics of lipid nanodomains on E. coli membranes during the course of AMR evolution, while high-resolution Atomic Force Microscopy provided complementary information on nanoscale morphological changes in the same population.
Our integrated approach revealed the onset of length scale–dependent dynamical changes in the membrane even at sub-MIC concentrations, suggesting that membrane reorganization is an early and adaptive response that facilitates survival at MIC. Furthermore, we detected signatures of cooperative lipid motion and dynamic heterogeneity in the membrane, quantified using the non-Gaussian parameter of lipid number fluctuations.
Together, these findings provide the first direct evidence linking nanoscale dynamical reorganization of Gram-negative bacterial membranes to the evolution of phenotypic resistance under sub-lethal exposure to colistin. By integrating RT-PCR, AFM, and STED-FCS measurements, we demonstrate how membrane remodeling serves as a key component of antibiotic resistance in E. coli.
For more information, see Parthasarathi, S., Chaudhury, A., Basu, J.K., Yadav, R. and Saini, D.K., 2025. Unraveling Direct Correlations between Membrane Nanodomain Reorganization and Antimicrobial Resistance Evolution in Bacterial Cells. PRX Life, 3(2), p.023017. https://doi.org/10.1103/4ksf-x7js
Harnessing Light for Advanced Quantum Technologies
What We Did
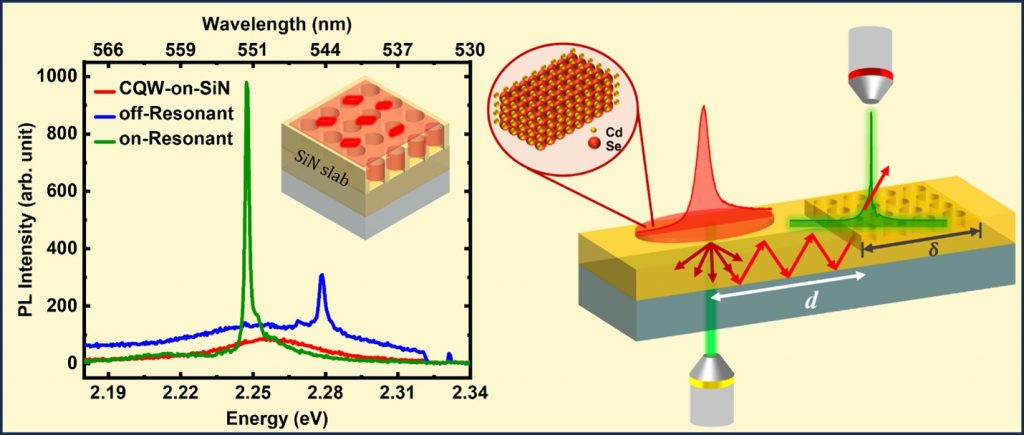
We’ve developed a method to enhance and purify light emission from 2D semiconductor colloidal quantum wells (CQWs) at room temperature. By integrating these tiny light sources with specially designed dielectric metasurface resonators (MSRs), we’ve created a system that emits pure light with high efficiency.
Key Findings
– The integration of CQWs with MSRs led to a significant enhancement and spectral purification of the light emitted by the CQWs.
– The significance of spectral overlap for efficient light-matter interaction was highlighted by demonstrating CQW coupling to MSRs with slightly de-tuned resonance responses.
– Spectrally narrowed emission was obtained in the guided in-plane direction too.
– We demonstrated that our setup can transport light over long distances on a chip, which is useful for creating on-chip light sources.
– Our experimental results were supported by theoretical and numerical models, explaining the data in terms of modified light-matter interactions (Lamb shifts and Purcell decays)
Why It Matters
Efficient and pure light sources are crucial for advancing technologies like quantum metrology and quantum cryptography, which have applications in secure communication and precise measurements. Our work could pave the way for new on-chip photonic devices that are faster and more reliable.
Reference: Room Temperature Emission Line Narrowing and Long-Range Photon Transport in Colloidal Quantum Wells Coupled to Metasurface Resonance, Advanced Optical Materials, https://doi.org/10.1002/adom.202400859
Spontaneous unbinding transition of nanoparticles adsorbing onto biomembranes: interplay of electrostatics and crowding
Cellular membranes are constantly bombarded with biomolecules and nanoscale particles, and cell functionality depends on the fraction of the bound/internalized entities. Understanding the bio-physical parameters underlying this complex process is very difficult in live cells. Model membranes provide an ideal platform to obtain insight into the minimal and essential parameters involved in determining cell membrane-nanoparticle (NP) interaction. Here we report spontaneous binding and unbinding of semiconductor NPs, carrying different net charges and interacting with model biomembranes, using in-situ neutron reflectivity (NR) and fluorescence microscopy studies. We observe a critical concentration of NPs above which they spontaneously unbind along with lipids from lipid monolayer membranes, leaving behind fewer bound NPs. This critical concentration varies depending on whether the NPs carry a net charge or are neutral, and is also governed by the extent of NP crowding for a fixed NP charge. The observations suggest a subtle interplay between electrostatics, membrane fluidity, and NP crowding effects which eventually determines the adsorbed concentration for unbinding transition. Our study provides valuable microscopic insight into the parameters that could determine the biophysical process underlying NP uptake & ejection by cells which, in turn, can be utilized for their potential application in bioimaging & drug delivery.



